In pharmaceutical manufacturing, maintaining sterile conditions and preventing contamination isn’t just a quality concern—it’s a regulatory requirement that directly impacts patient safety. Every connection point in your processing system represents a potential entry point for contaminants, making sanitary connections one of the most critical aspects of pharmaceutical facility design and operation.
The Critical Role of Sanitary Connections
Pharmaceutical manufacturing operates under some of the strictest regulatory standards in any industry. The FDA, EMA, and other regulatory bodies mandate rigorous controls over every aspect of production, from raw material handling to final packaging. Sanitary connections serve as the foundation for maintaining these standards throughout your facility.
Unlike standard industrial connections, sanitary connections are designed to eliminate dead spaces, crevices, and surface irregularities where bacteria, particles, or cleaning residues could accumulate. These design features are essential for maintaining the sterile environment required for pharmaceutical production while enabling effective cleaning and sterilization procedures.
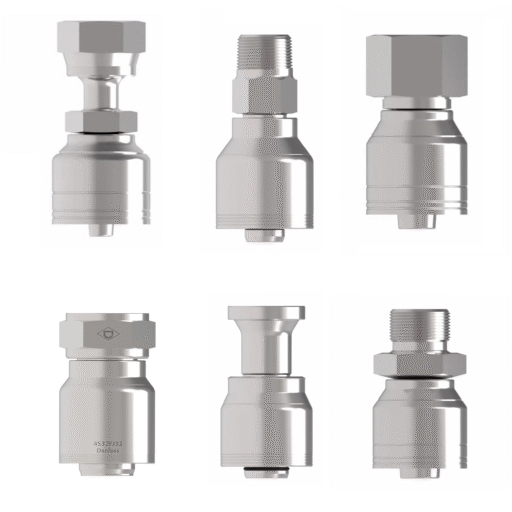
Foster Hose & Fittings specializes in 3-A certified sanitary assemblies that meet the stringent requirements of pharmaceutical manufacturing. Our sanitary hose systems feature industry-best 3-A sanitary connections that provide reliable, leak-free performance while maintaining the cleanliness standards your processes demand.
Understanding 3-A Sanitary Standards
The 3-A Sanitary Standards represent the gold standard for sanitary equipment design in pharmaceutical and food industries. These standards specify precise requirements for materials, surface finishes, drainage, and cleanability that ensure equipment can be effectively sanitized between production runs.
Our RubberworxTM sanitary hose assemblies are built to exceed 3-A standards, featuring smooth interior surfaces that resist bacterial adhesion and facilitate complete cleaning. The white FDA nitrile tube construction provides chemical resistance while maintaining the purity requirements essential for pharmaceutical applications.
The 3-A certification process involves rigorous testing and validation to ensure equipment meets all cleanability and sanitization requirements. This certification provides the documentation needed to support regulatory compliance and quality assurance programs in pharmaceutical facilities.
Material Selection for Pharmaceutical Applications
Material selection plays a crucial role in sanitary connection performance. Pharmaceutical applications require materials that resist chemical attack from cleaning agents and active pharmaceutical ingredients while maintaining their properties through repeated sterilization cycles.
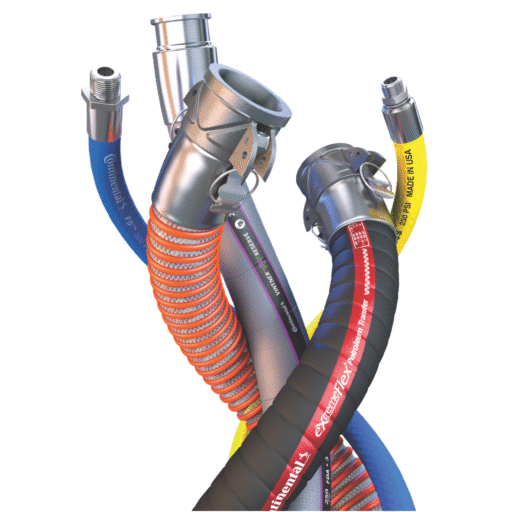
Our sanitary hose assemblies feature FDA-approved materials throughout the entire flow path. The white FDA nitrile tube provides excellent chemical resistance and maintains its properties through multiple cleaning and sterilization cycles. The 2-ply textile and wire helix reinforcement provides structural integrity while maintaining flexibility for easy installation and routing.
Stainless steel components meet pharmaceutical-grade requirements for surface finish and chemical resistance. The precision manufacturing ensures smooth surfaces that resist bacterial adhesion while providing the mechanical strength needed for repeated assembly and disassembly during maintenance operations.
Temperature Requirements and Sterilization Compatibility
Pharmaceutical manufacturing often involves elevated temperatures for sterilization processes, heating applications, and cleaning procedures. Our sanitary hose systems are designed to handle temperatures from -25°F to 230°F, providing the flexibility needed for diverse pharmaceutical applications.
Steam sterilization represents one of the most demanding thermal cycles in pharmaceutical manufacturing. Our assemblies maintain their integrity and performance through repeated steam sterilization cycles, ensuring reliable operation throughout their service life. The temperature stability prevents degradation that could compromise cleanliness or introduce contaminants into the process stream.
Cold storage and cryogenic applications also benefit from the temperature range capabilities of our sanitary systems. The flexibility at low temperatures ensures reliable operation even in frozen storage applications or when handling temperature-sensitive pharmaceutical ingredients.
Color-Coding for Contamination Prevention
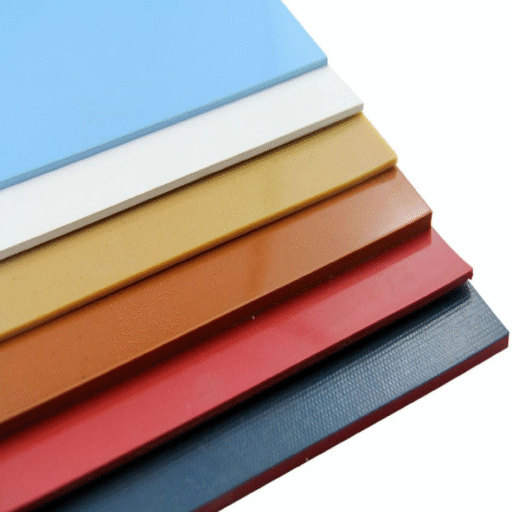
Visual identification through color-coding provides an additional layer of contamination prevention in pharmaceutical facilities. Our RubberworxTM sanitary hoses are available in six different colors: white, gray, red, blue, green, and orange. This variety enables comprehensive color-coding systems that prevent cross-contamination between different product lines, processing stages, or cleaning applications.
Color-coding proves especially valuable in facilities producing multiple pharmaceutical products or handling both active ingredients and excipients. The visual identification helps ensure the correct hose is used for each application while supporting cleaning validation and change-over procedures.
The fade-resistant colors maintain their clarity throughout the service life of the hose, ensuring the color-coding system remains effective over time. This reliability supports long-term contamination prevention strategies while reducing the need for frequent hose replacement due to color degradation.
Cleaning and Sterilization Procedures
Effective cleaning and sterilization procedures are essential for maintaining sanitary conditions in pharmaceutical manufacturing. The design of sanitary connections directly impacts the effectiveness of these procedures by eliminating areas where cleaning solutions cannot reach or where residues could accumulate.
Our sanitary hose assemblies feature smooth interior surfaces that facilitate complete cleaning and drainage. The lack of dead spaces or crevices ensures cleaning solutions can reach all surfaces while preventing the accumulation of residues that could harbor contaminants or interfere with subsequent processes.
The chemical resistance of our assemblies allows compatibility with aggressive cleaning agents commonly used in pharmaceutical facilities. This resistance ensures the hose maintains its properties and performance even when exposed to strong acids, bases, or sanitizing solutions during routine cleaning procedures.
Validation and Documentation Requirements
Pharmaceutical manufacturing requires extensive documentation to support regulatory compliance and quality assurance programs. Our sanitary hose assemblies come with complete documentation packages that include material certifications, test results, and traceability information.
Material certifications verify that all components meet FDA requirements and other applicable standards. This documentation supports ingredient master files and regulatory submissions while providing the traceability required for quality management systems.
Testing documentation includes pressure testing results, dimensional verification, and cleanliness certification. This information supports installation qualification and operational qualification procedures required for pharmaceutical equipment validation.
High Purity Transfer Applications
Many pharmaceutical processes involve transferring high-purity materials where even trace contamination could compromise product quality or patient safety. Our sanitary hose systems provide the clean transfer capabilities these applications require.
The smooth interior surface prevents particle generation that could contaminate high-purity streams. The chemical inertness of our materials ensures no extractables or leachables compromise product purity. These characteristics prove essential for applications involving purified water, water for injection, or high-purity pharmaceutical ingredients.
The permanent crimp end connections eliminate potential leak points while maintaining the sanitary characteristics of the system. This design ensures product integrity throughout the transfer process while supporting the cleanliness requirements of pharmaceutical manufacturing.
Installation and Maintenance Considerations
Proper installation practices are essential for maintaining the sanitary characteristics of connection systems. Our technical team provides guidance on installation procedures that preserve the cleanliness and performance of sanitary assemblies.
Proper support and routing prevent stress concentrations that could lead to premature failure or create cleaning challenges. The flexibility of our assemblies allows routing around obstacles while maintaining the integrity needed for pharmaceutical applications.
Maintenance procedures should account for the sanitary requirements of pharmaceutical manufacturing. Our assemblies are designed for easy removal and replacement during scheduled maintenance while maintaining system cleanliness throughout the process.
Regulatory Compliance and Quality Assurance
Pharmaceutical manufacturing operates under strict regulatory oversight that requires comprehensive quality assurance programs. Our sanitary hose assemblies support these requirements through design features and documentation that facilitate compliance.
The 3-A certification provides regulatory recognition of sanitary design principles while our FDA-approved materials ensure compliance with food and drug regulations. This combination supports regulatory submissions and inspections while providing confidence in system performance.
Our quality management system ensures consistent manufacturing processes that deliver reliable performance. Statistical process control and lot tracking provide the documentation needed to support pharmaceutical quality systems while ensuring product consistency.
Custom Solutions for Specialized Applications
While standard sanitary assemblies handle most pharmaceutical applications, some processes require custom solutions to meet unique requirements. Our engineering team works with customers to develop specialized assemblies that maintain sanitary characteristics while addressing specific operational needs.
Custom length assemblies eliminate unnecessary connections that could compromise cleanliness or create potential leak points. Special end configurations accommodate unique equipment interfaces while maintaining sanitary design principles.
Multi-line assemblies reduce installation complexity while maintaining individual line integrity. This approach proves valuable in facilities with multiple parallel processes or where space constraints limit individual line routing options.
Cost-Effectiveness Through Reliability
Sanitary connections represent a critical investment in pharmaceutical manufacturing quality and compliance. While high-quality assemblies require higher initial investment, their reliability and performance often result in lower total cost of ownership through reduced maintenance, fewer change-outs, and improved process reliability.
The cost of contamination incidents, product recalls, or regulatory actions far exceeds the investment in quality sanitary connections. Our assemblies provide the reliability needed to prevent these costly events while supporting consistent production operations.
Our technical expertise helps customers select optimal solutions for their specific applications, ensuring proper performance while avoiding over-specification that could increase costs unnecessarily.
Frequently Asked Questions
Q: What’s the difference between 3-A certified and FDA-approved materials in sanitary connections?
FDA approval covers material safety for food and drug contact, while 3-A certification addresses the sanitary design principles needed for effective cleaning and sterilization. Our assemblies meet both requirements for comprehensive compliance.
Q: How often should sanitary hose assemblies be replaced in pharmaceutical applications?
Replacement frequency depends on usage patterns, cleaning cycles, and operating conditions. Regular inspection and testing help determine optimal replacement intervals while maintaining system integrity and compliance.
Q: Can sanitary connections handle both liquid and powder pharmaceutical ingredients?
Yes, our sanitary assemblies are designed for versatile pharmaceutical applications. The smooth interior surface works well for both liquid transfer and powder handling while maintaining cleanliness requirements.
Q: What cleaning validation considerations apply to sanitary hose assemblies?
Cleaning validation should verify that cleaning procedures effectively remove all product residues and cleaning agents. Our smooth interior surfaces and proper drainage characteristics support effective cleaning validation protocols.
Q: How do I ensure proper storage of sanitary hose assemblies before installation?
Store assemblies in clean, dry conditions with end caps in place to prevent contamination. Avoid exposure to UV light or extreme temperatures that could affect material properties before installation.
Q: What documentation is provided with pharmaceutical-grade sanitary assemblies?
We provide complete documentation packages including material certifications, dimensional reports, pressure test results, and traceability information to support pharmaceutical quality systems and regulatory requirements.
For pharmaceutical manufacturing applications where product integrity and regulatory compliance are non-negotiable, Foster Hose & Fittings provides the sanitary connection solutions your operations require. Contact our pharmaceutical specialists at (314) 947-5799 to discuss how our 3-A certified sanitary assemblies can support your manufacturing processes while ensuring the highest standards of cleanliness and compliance.